A retrospective study of the impact of comorbidity, polypharmacy and demographic factors on patient inclusion and healthcare delivery in phase I oncology trials
Comorbidity refers to the presence of two or more chronic conditions within an individual and poses significant challenges for healthcare systems [1]. In the UK, patients with comorbidity account for most primary care consultations, prescriptions, and hospitalisations [2, 3]. The higher risk of comorbidity in cancer patients results in the risk of polypharmacy and its detrimental impact on their quality of life [4]. Hence, examining polypharmacy along with comorbidity in cancer patients is crucial. Researchers focused on the impact of comorbidity and polypharmacy on the management of patients with a range of cancers and found that either comorbidity, polypharmacy, or both reduced the chance or delayed the institution of systemic anticancer therapy and, in some cases, was correlated to shorter survival [5,6,7].
Clinical trials are crucial in cancer care. Primarily, to ensure a robust assessment of safety, efficacy, and utility, participants in cancer clinical trials should be representative of the target population. However, comorbidity is prevalent in the population of cancer patients, and it is therefore crucial that cancer patients with comorbidity, who are a complex group with specific health service needs, are considered in trials. Due to the fact that most clinical trials are designed with a single disease in mind, and there is a risk of concealing the potential benefits of experimental treatment, performing such clinical trials has been limited [8,9,10,11]. Investigating comorbidity in cancer trial recruitment has rarely been studied. Unger et al. [9] investigated whether clinical trial decision-making and participation were linked to comorbidity in patients with cancer. The study conducted a web-based survey of patients with cancer and assessed multiple recruitment-related outcomes, including discussion of participation, offer of participation, and participation. They found that all the outcomes were negatively impacted by comorbidity.
Phase I trials are conducted primarily to recommend a safe dose/schedule of novel anticancer drugs. These trials are conducted in patients with metastatic cancer who have previously received standard-of-care treatments for their disease and often have significant cancer-related morbidity. Previous studies have explored the impact of sociodemographic factors on recruitment to phase I cancer trials [12, 13]. However, they have not examined the role of comorbidity and polypharmacy in this context. Additionally, the effect of these clinical factors on health service utilisation during trials remains largely unaddressed. We aimed to study if there is evidence that polypharmacy, comorbidity, and sociodemographic features (age, sex, ethnicity, distance to the hospital, and index of multiple deprivation (IMD)) are associated with phase I cancer trial recruitment. Further, we investigated if these factors interact with the experimental treatment to affect other healthcare service use, namely, disease progression through an increase in the discontinuation rate or the number of emergency scans or admissions and length of stay post-admission.
Methods
Study population and data
This retrospective study used electronic patient record (EPR) data from cancer patients referred to the Drug Development Unit (DDU), a joint department at the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research from their local hospitals. All patients seen in the referred and seen in the new patient clinics and subsequently discussed for consideration of recruitment to a phase I trial in the patient allocation meeting (PAM) within 28 days of being seen between 01/10/2018 and 31/12/2021 were considered for analysis. Patients were deemed to have started a phase I study if they had received at least one drug dose. Data on individual patients were collected on the date they were first seen in the new patient clinic and if they were recruited to the phase I trial till the last date they were seen on the trial. If the patient was not allocated or was allocated and found ineligible, data was collected till the last recorded clinic visit in the DDU.
The base dataset was collected from patients on the DDU patient list. It included data from trial enrolment, trial start date, trial end date, death, clinic visit date, age at the time of referral, sex, ethnicity, IMD [14], distance to the Royal Marsden Hospital – Sutton, the number of medications that were not directly used to treat cancer, i.e., excluding chemotherapy, hormonal therapy or immunotherapy, or investigational drug in the clinical trial, the number of diseases, emergency admissions, and emergency scans.
We filtered the dataset based on the closeness of the last clinic visit to the PAM and trial start dates. The threshold to determine the cohort for patients being considered for recruitment was 28 days from the last clinic visit and PAM date. The threshold to determine the trial group was 120 days from the last clinic visit and trial start date. It is worth noting that the threshold for the trial group was larger than that of the recruitment cohort, as we were more concerned with the clinician’s sensitivity to the patient’s baseline characteristics in the decision to recruit into the trial.
Data analysis
In our analysis, independent variables comprised sociodemographic features and health-related variables. Sociodemographic features included age at the first clinic appointment in the DDU, sex, ethnicity, IMD score, and distance to the Royal Marsden Hospital – Sutton in miles. Ethnicity was initially recorded in line with the NHS ethnic categories, resulting in small sample sizes for each group. Hence, we recorded this variable as white and non-white groups. Due to non-disclosure, ten missing observations for the ethnicity variable were imputed using the multiple imputation by chained equations algorithm. The IMD was used as a surrogate for income deprivation and had the following components (weighting in brackets): income (22.5%), employment (22.5%), health deprivation and disability (13.5%), education and skills training (13.5%), crime (9.3%), barriers to housing and services (9.3%), and living environment (9.3%). Higher scores for IMD indicate greater deprivation and are assigned from the postcode at registration. Also, distance to the hospital was calculated using patients’ postcodes.
Health-related variables included polypharmacy and comorbidity. There were several options concerning the variable type for polypharmacy and comorbidity. Comorbidity is often quantified using indices such as the Charlson comorbidity index [15] and the Elixhauser comorbidity index [16], primarily developed as prognostic tools. However, both indices apply weighting schemes based on long-term mortality risk, which may not be directly applicable or valid in early-phase oncology trials, where short-term safety and tolerability are the primary endpoints. We chose the number of medications as a surrogate for polypharmacy, which was a simple and reliable variable. Similarly, we initially opted for the number of diseases as a surrogate for comorbidity; however, that resulted in small sample sizes for each group. Hence, comorbidity was treated as a dichotomous variable, indicating the presence or absence of at least one non-cancer condition in individuals with cancer. It is worth noting that in the remainder of this report, we refer to comorbidity as the dichotomous variable.
Both the number of medications and the number of diseases were extracted from free-text patients’ case notes. Disease data was typically in paragraphs, with superfluous data including family medical history and health behaviours. Hence, string manipulation and stop word removal were used to reduce text. The algorithmic extraction of diseases was unfeasible due to synonyms, acronyms, and possible typos. Hence, this was done manually, and the extracted list was validated by clinicians. The extracted diseases were then summed per patient and then grouped into dichotomous for the comorbidity variable. Medications were explicitly listed rather than nested in free text, and punctuation separated terms. There were issues with superfluous text, and the inclusion of complementary medication was deemed unimportant. String manipulation was used to remove additional text, and fuzzy matching was deployed to extract the medications per patient. Medication data was extracted from clinical notes within the patients’ EPR. As such, the dataset may not capture medications prescribed by general practitioners (GPs) or any over-the-counter medications taken by patients. Additionally, it is worth noting that only medicines included in the British National Formulary [17] were extracted.
The outcome variables included recruitment into a clinical trial, time on trial, the number of emergency scans, the number of emergency admissions, and the number of days in the hospital associated with those emergency admissions. The number of emergency admissions and scans collected from patient case notes, and the EPR involved a small set of terms; hence, rules-based algorithms were employed. In patients’ case notes, the data was collected during DDU clinic visits at the patient’s discretion. In the EPR, admissions and scans that were not routine were flagged as emergency. Scans contributed to the number of scans variable, including CT, MRI, PET, X-ray, and ultrasound.
Independent variables were either binary or continuous. Comorbidity, sex, and ethnicity were recorded as binary variables; the number of medications, the number of diseases, age, distance to the Royal Marsden Hospital – Sutton, and IMD score were recorded as continuous variables. The outcome variables were split into binary, continuous, and time-to-event variables. Trial recruitment was coded as a binary variable; the number of emergency admissions, emergency scans, and length of stays after admissions were considered continuous variables, and time on trial was regarded as a time-to-event variable. An event was defined as either having disease progression or death led to a withdrawal.
We aimed to produce statistical models that estimated estimands with minimal bias and consistency. To do so, we needed a directed acyclic graph (DAG) to define the variables required to identify the estimands. Following the guidance from Rodrigues et al. [18] and with the assistance of clinicians, the DAG was defined (supplementary). The DAG included additional observed and unobserved variables to find the adjustment set that fulfilled the backdoor criterion [19]. As validated by dagitty.com [20], the adjustment set involved all independent variables, with additional variables being unadjusted as they were deemed colliders. The DAG was considered general for our outcomes, so the adjustment set was carried over into each statistical model.
Statistical analysis
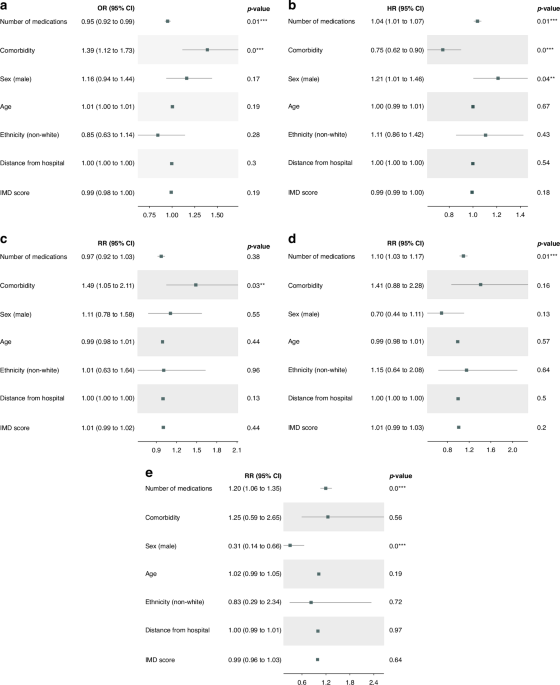
Regression models were used to explore the effect of independent variables on the outcome variables. The analysis was carried out in both univariate and multivariable settings. It is worth noting that in the univariate analysis, the number of diseases and comorbidity were investigated to conduct a comprehensive analysis. However, one of them had to be chosen for the multivariable analysis. Hence, comorbidity was considered for the multivariable analysis. Also, before conducting the primary analysis, we examined whether the number of medications and diseases interacted.
For trial recruitment, as a binary variable, a logistic regression model was used, and odds ratios (ORs) were reported. For the number of emergency admissions, emergency scans, and length of stays after admissions, as continuous variables, Poisson and negative binomial were considered according to the regression-based test for overdispersion [21], and risk ratios (RRs) were reported. Since we found evidence of overdispersion in the number of scans and length of stays, these models were fitted with negative binomial models. Also, the number of emergency admissions variable was fitted with a Poisson model. For time on treatment, as a time-to-event variable, we tested and found evidence for proportional hazards. Hence, a Cox proportional hazards model was fitted, and hazard ratios (HRs) were reported.
link







